Abstract
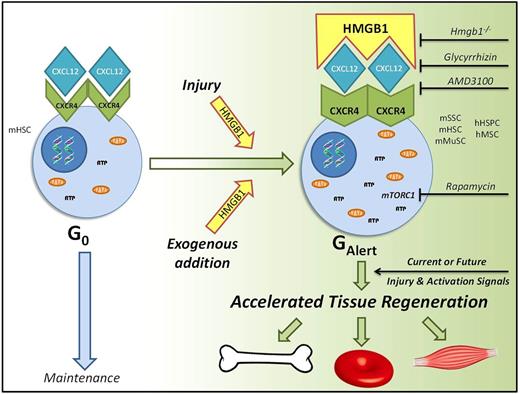
Chemotherapy induced leucopoenia and neutropaenia results in an increased risk of infection, with febrile neutropaenia being associated with bacteraemia, sepsis and death. Haematopoietic regeneration is reliant on endogenous stem cells and the acceleration of this process would reduce the length of time (nadir) of clinical neutropaenia, thereby reducing the risk of infection and the treatment associated morbidity and mortality associated with chemotherapy. The haematopoietic stem cell injury-niche has not been extensively explored for potential novel in vivo regenerative targets. Our objective was to assess if alarmins, endogenous molecules which are released upon tissue damage and widely considered to only be triggers of the immune response, may potentiate stem-cell mediated repair and regeneration. Here we show that the alarmin HMGB1 transitions endogenous quiescent G0 stem cells to the recently described GAlert phase, thereby enhancing regeneration in multiple tissues, including blood, bone, and muscle.
Utilising complete diaphyseal fractures as a model of massive HSC-niche injury, we found that alarmins were elevated post-fracture in both human and murine blood samples. Next, we administered exogenous HMGB1 systemically to wild type C57BL6/J mice and serial peripheral blood counts showed they displayed accelerated haematopoietic recovery following 5-fluouracil (5-FU) induced myeloablation. The nadirs of leucopoenia and neutropaenia were reduced by approximately 28% and 23% respectively. Analysis of cell cycle kinetics, cell size, ATP levels, mitochondrial DNA, and mTORC1 dependency revealed that the underlying mechanism was that HMGB1 transitioned the murine haematopoietic stem cell to the dynamic GAlert phase. We confirmed these findings by generating conditional Hmgb1-/- mice and performed rescue experiments. Additionally we showed these cellular effects extended to CD34+ human haematopoietic stem and progenitor cells.
Hybrid ELISAs and small molecule inhibitors showed that the molecular pathway through which HMGB1 exerted these regenerative effects was by forming a heterocomplex with CXCL12 and acting via CXCR4. Subsequently we showed that other CXCR4+ stem and progenitor cells, including murine skeletal and muscle stem cells, and human mesenchymal stromal cells, also transitioned to GAlert following administration of exogenous HMGB1. HMGB1 also accelerated fracture repair and muscle regeneration, and accelerated recovery in all three murine models when administered 2 weeks before injury. In summary, we have identified a molecular and cellular pathway that accelerates the physiological regenerative response in a dynamic and adaptive manner to current or future injuries. We propose a model in which a highly-conserved injury signal, HMGB1, acts via a well-established maintenance signalling pathway, CXC12-CXCR4, to ultimately promote tissue regeneration. As a therapeutic target with broad clinical applications, such as in chemotherapy, trauma, or elective surgery, FR or 3S-HMGB1 could be administered as a single dose either locally or systemically at the time of injury or even up to 2 weeks beforehand. Most importantly this pathway has wide potential therapeutic benefit, as it could conceivably accelerate healing in any tissue that relies on repair from cells that express CXCR4 and can transition to GAlert.
Venereau: HMGBiotech: Employment, Patents & Royalties: HMGBiotech. Bianchi: HMGBiotech: Employment, Patents & Royalties: HMGBiotech.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal